Regulating Combination Products in 2025
What Are Combination Products and Why They Matter in 2025
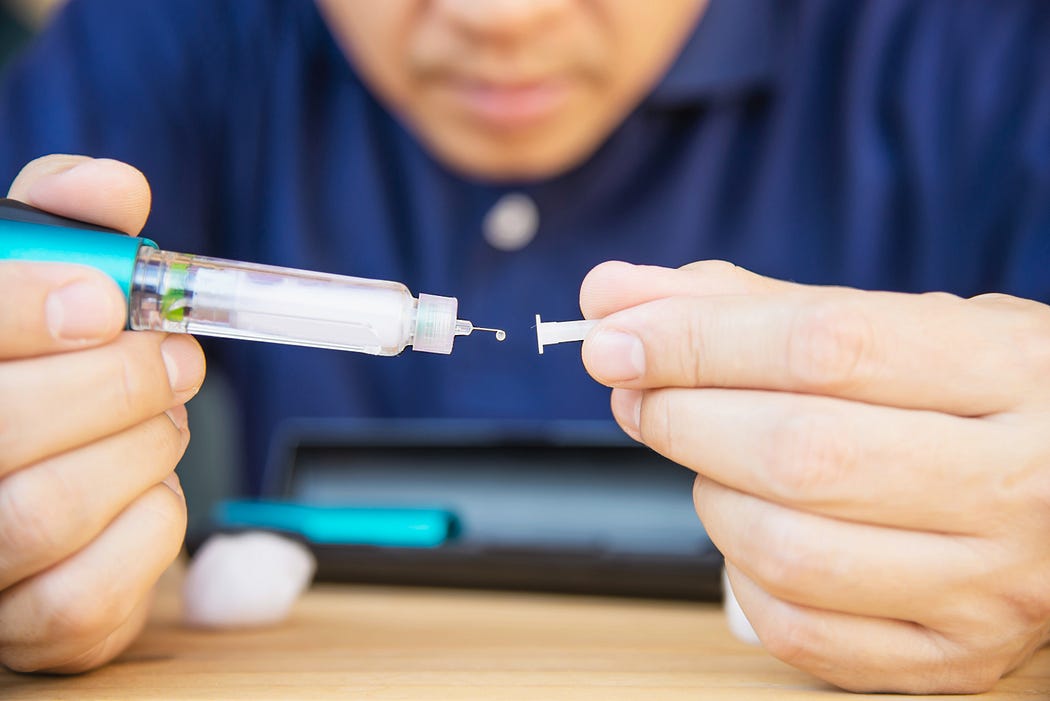
Combination products, where a medical device, drug, or biologic come together to deliver a single therapeutic effect, are redefining how modern medicine reaches patients. From insulin pens and drug-eluting stents to AI-enabled inhalers, these hybrids represent the next frontier in precision treatment and patient convenience.
The global drug-device combination market was valued at USD 138.48 billion in 2023 and is projected to reach USD 251.9 billion by 2030, growing at a 9% CAGR. Nearly 30% of all medical product filings now involve some form of drug-device combination. These numbers reflect both scientific progress and regulatory complexity.
Despite years of harmonization efforts, each region continues to regulate the combination of products through distinct frameworks, definitions, and review mechanisms. For global manufacturers, the real challenge is no longer scientific feasibility, but regulatory navigation.
Read more from DDReg Experts here: Combination Products in 2025: Regulatory Convergence or Compounding Complexity? — DDReg pharma

Comments
Post a Comment